selective treatment of acne symptoms
Derma AKNE
 Derma AKNE is a complex selective acne symptom improvement equipment using IPL and RF technology.
Derma AKNE is a complex selective acne symptom improvement equipment using IPL and RF technology.


Acne treatment must now destroy sebaceous glands.
Derma AKNE received approval from the Ministry of Food and Drug Safety regarding the
“improvement of moderate and heavy acne symptoms in adults.” 1/28/2021

Derma AKNE’s effectiveness regarding facial acne treatment was proven,
receiving the CE mark and ISO13485 certification as a composite medical device.
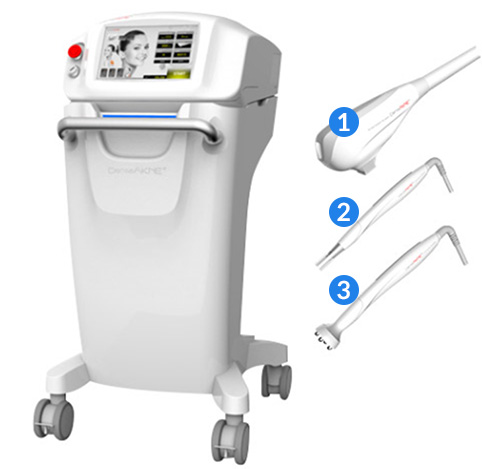
Details about how the device works 
Shape and structure - how the devices works
-
01

General purpose electro-surgical unit
This product is composed of the product body, a monopolar handpiece, Bovie plate, foot switch power cable, and single-time-use foot-controlled electro-surgical electrodes (medical certification number 17-4119). Such products use radio frequency energy and the heat produced by the load or contact resistance to cause coagulation in cell structures.
-
02

High frequency stimulator
The high frequency stimulator produces heat in the affected area when the high frequency energy enters the body, reducing pain. The high frequency energy makes the molecules vibrate whenever the current switches direction, causing friction; the resulting spinning, distortion, and collisions produce body heat.
-
03
Intense pulsed light device
This device uses the xenon flash lamp in the hand piece to create wavelengths of 400~1000nm and shine them on the parts of the body being treated, enhancing the treatment of skin disorders. The hand piece transfers light energy to the body as heat energy. The pulse waves are absorbed by chromophores that react to certain wavelengths to produce heat, which destroys those chromophores; this is the working principle of the device.
-
04

Improves moderate/severe acne in adults
This product uses software to control and selectively apply radio frequency energy from the general purpose electro-surgical unit and the intense pulsed light device’s pulse waves on acne-affected skin, improving moderate and heavy acne symptoms.
The general purpose electro-surgical unit mode uses partially insulated single-time-use foot-controlled electro-surgical electrodes (medical certification number 17-4119) to cause a pyrolyzing effect on the true skin layer of areas affected by acne without damaging the epidermis or the upper derma to selectively destroy only the sebaceous glands. This reduces the creation of sebum that serves as a medium for acne germs and uses direct heat to destroy such germs.
“Only the sebaceous glands are selectively destroyed, reducing the creation of sebum which serves as a medium for the grown of acne germs. Direct heat destroyed the germs. themselves.” The intense pulsed light device mode shines light energy from the xenon lamp on porphyrin created by acne germs, which creates active oxygen. This active oxygen destroys the cell walls of the acne germs, killing them.
-
1 Soft Vacuum IPL
-
2 Needle RF
-
3 Bipolar Melting RF
-

Soft Vacuum IPLDestruction of the germs that cause acne
The intense pulsed light created by the intense pulsed light device creates negative pressure on the skin. The light then destroys the acne germs directly.
-

Needle RFSelective destructive of sebaceous glands
this device selectively destroys sebaceous glands, preventing the recurrence of acne.
-

Bipolar Melting RFAlleviation of acne infection, sebum discharge, enhanced regeneration
the high frequency stimulator uses the high frequency heat created by the general purpose electro-surgical unit to make sebum easier to discharge.
 Product inquiries
Product inquiries
 82-80-608-7000
82-80-608-7000
Please leave any inquiries you have about Derma AKNE, and we will address your inquiry as quickly as possible.